Engineered Biosynthesis of beta-Alkyl Tryptophan Analogues.
Boville, C.E., Scheele, R.A., Koch, P., Brinkmann-Chen, S., Buller, A.R., Arnold, F.H.(2018) Angew Chem Int Ed Engl 57: 14764-14768
- PubMed: 30215880
- DOI: https://doi.org/10.1002/anie.201807998
- Primary Citation of Related Structures:
6CUT, 6CUV, 6CUZ - PubMed Abstract:
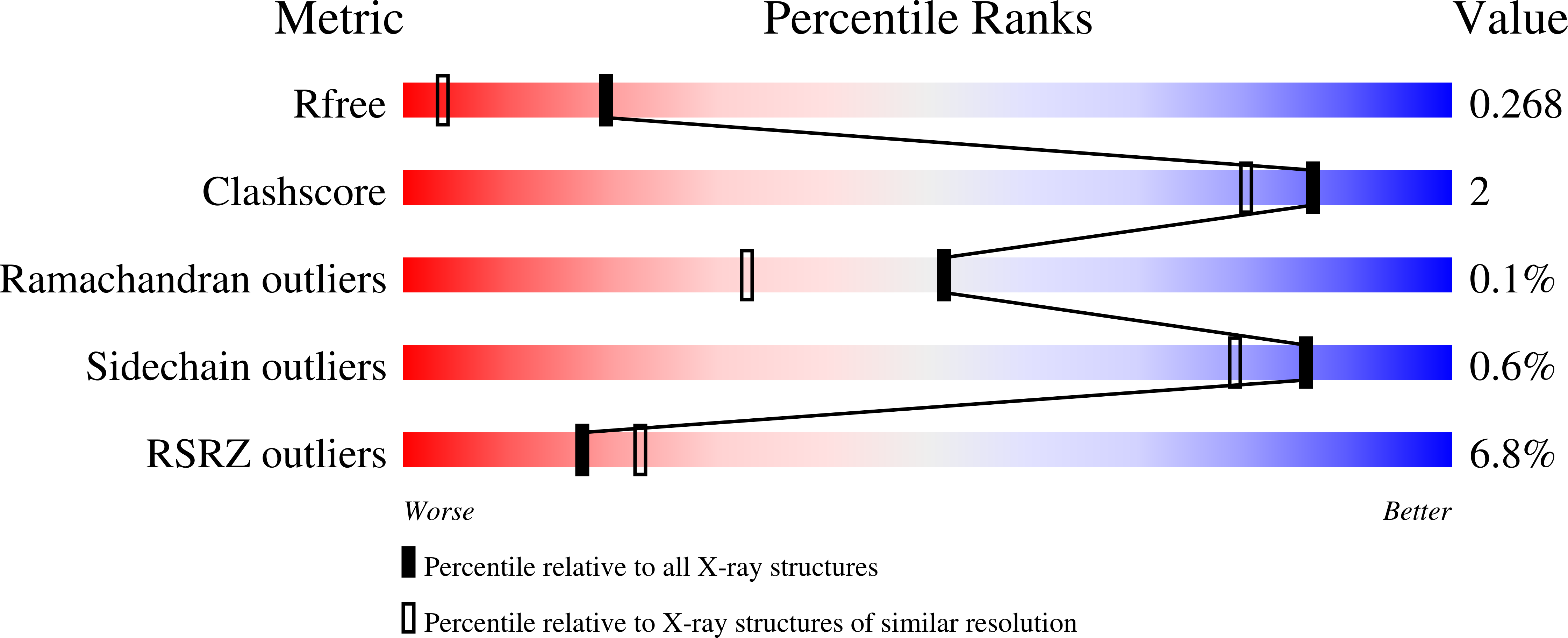
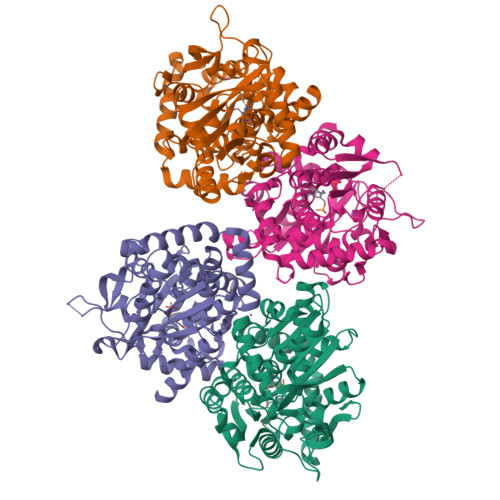
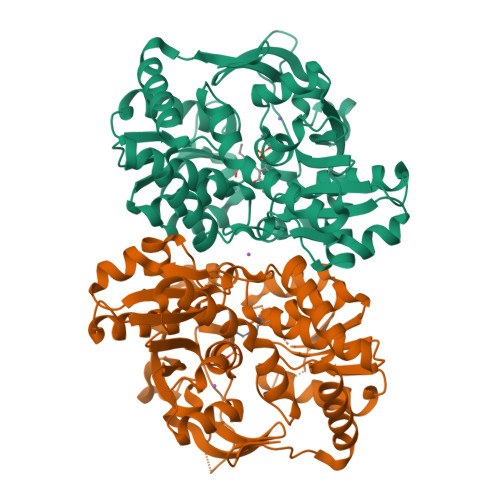
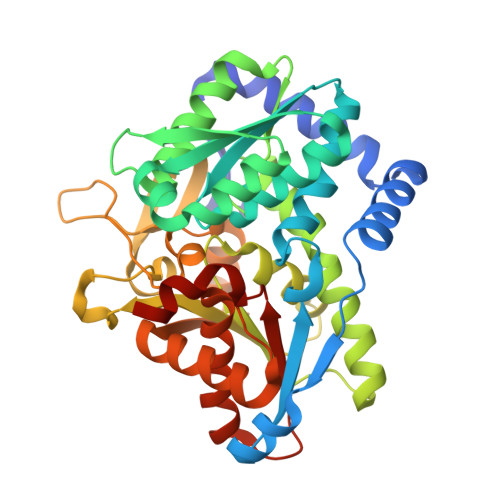
Noncanonical amino acids (ncAAs) with dual stereocenters at the α and β positions are valuable precursors to natural products and therapeutics. Despite the potential applications of such bioactive β-branched ncAAs, their availability is limited due to the inefficiency of the multistep methods used to prepare them. Herein we report a stereoselective biocatalytic synthesis of β-branched tryptophan analogues using an engineered variant of Pyrococcus furiosus tryptophan synthase (PfTrpB), PfTrpB 7E6 . PfTrpB 7E6 is the first biocatalyst to synthesize bulky β-branched tryptophan analogues in a single step, with demonstrated access to 27 ncAAs. The molecular basis for the efficient catalysis and broad substrate tolerance of PfTrpB 7E6 was explored through X-ray crystallography and UV/Vis spectroscopy, which revealed that a combination of active-site and remote mutations increase the abundance and persistence of a key reactive intermediate. PfTrpB 7E6 provides an operationally simple and environmentally benign platform for the preparation of β-branched tryptophan building blocks.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering 210-41, California Institute of Technology, 1200 East California Boulevard, Pasadena, California, 91125, USA.