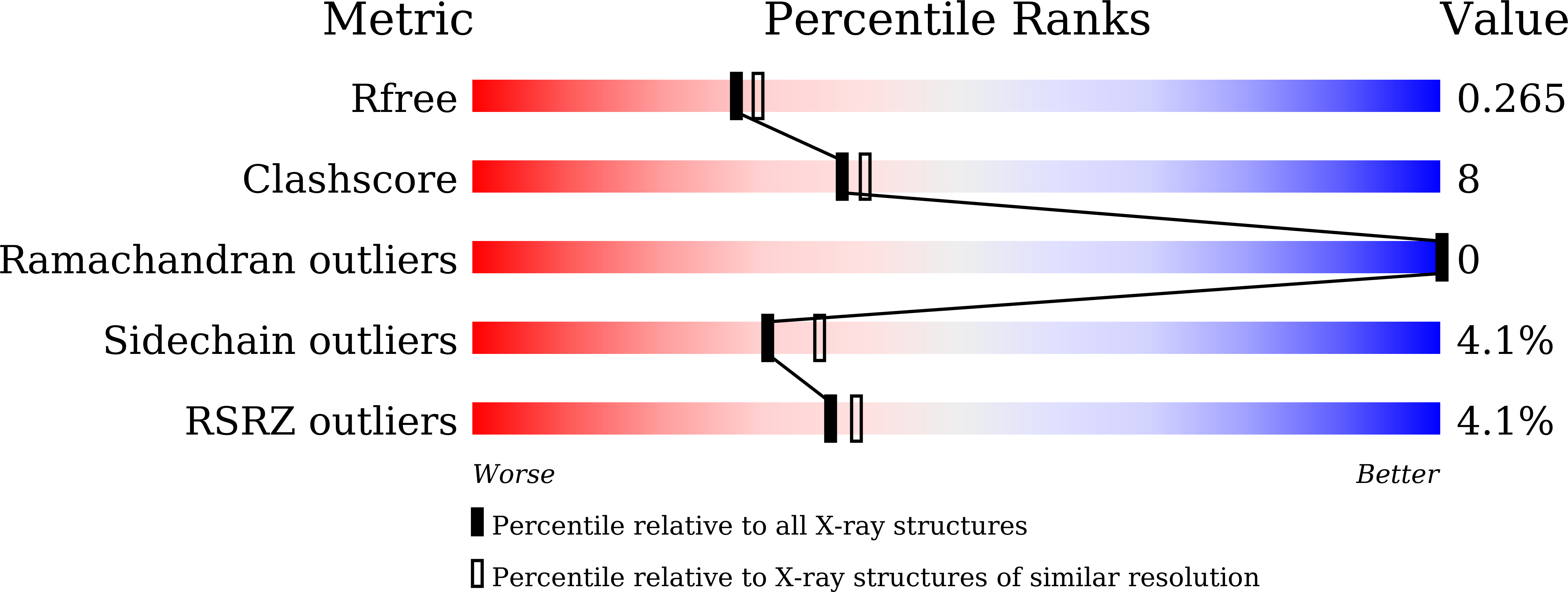
New insights into the substrate inhibition of human 17 beta-hydroxysteroid dehydrogenase type 1.
Li, T., Song, X., Stephen, P., Yin, H., Lin, S.X.(2023) J Steroid Biochem Mol Biol 228: 106246-106246
- PubMed: 36634828
- DOI: https://doi.org/10.1016/j.jsbmb.2023.106246
- Primary Citation of Related Structures:
7X3Z - PubMed Abstract:
Human type 1 17β-hydroxysteroid dehydrogenase (17β-HSD1),a member of the short-chain dehydrogenase/reductase family, catalyzes the last step in the bioactivation of the most potent estrogen estradiol with high specificity and is thus involved in estrogen-dependent diseases. As an oxidoreductase, 17β-HSD1 can utilize both triphosphate and diphosphate cofactors in reaction at the molecular level, but more specific with triphosphate cofactor. The NADPH is much higher than NADP+ in living cells leading to preliminary reduction action. The enzyme also showed substrate-induced inhibition unprecedented in other members of 17β-HSDs. Our previous study elucidated the structural mechanism of substrate inhibition is due to the reversely bound estrone (E1) in the substrate-binding pocket of the enzyme resulting in a dead-end complex. However, the effect of the cofactor preference on the substrate inhibition of the enzyme is not yet clear. In the present study, we solved the ternary crystal structures of 17β-HSD1 in complex with E1 and cofactor analog NAD+ . Combined with molecular dynamics simulation using the enzyme with NADH/NADPH and different oriented E1 (normally oriented, E1N; reversely oriented, E1R), such ternary structure provides a complete picture of enzyme-substrate-cofactor interactions. The results reveal that different cofactors and substrate binding mode affect the allosteric effect between the two subunits of the enzyme. And the results from MD simulations confirmed that His 221 plays a key role in the formation of dead-end complex in NADPH complex, and the absence of stable interaction between His 221 and E1R in the NADH complex should be the main reason for its lack of substrate inhibition.
Organizational Affiliation:
Dalian Engineering Research Center for Carbohydrate Agricultural Preparations, Liaoning Provincial Key Laboratory of Carbohydrates, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China; CHU de Québec Research Center and Department of Molecular Medicine, Laval University, Québec, QC, Canada. Electronic address: [email protected].