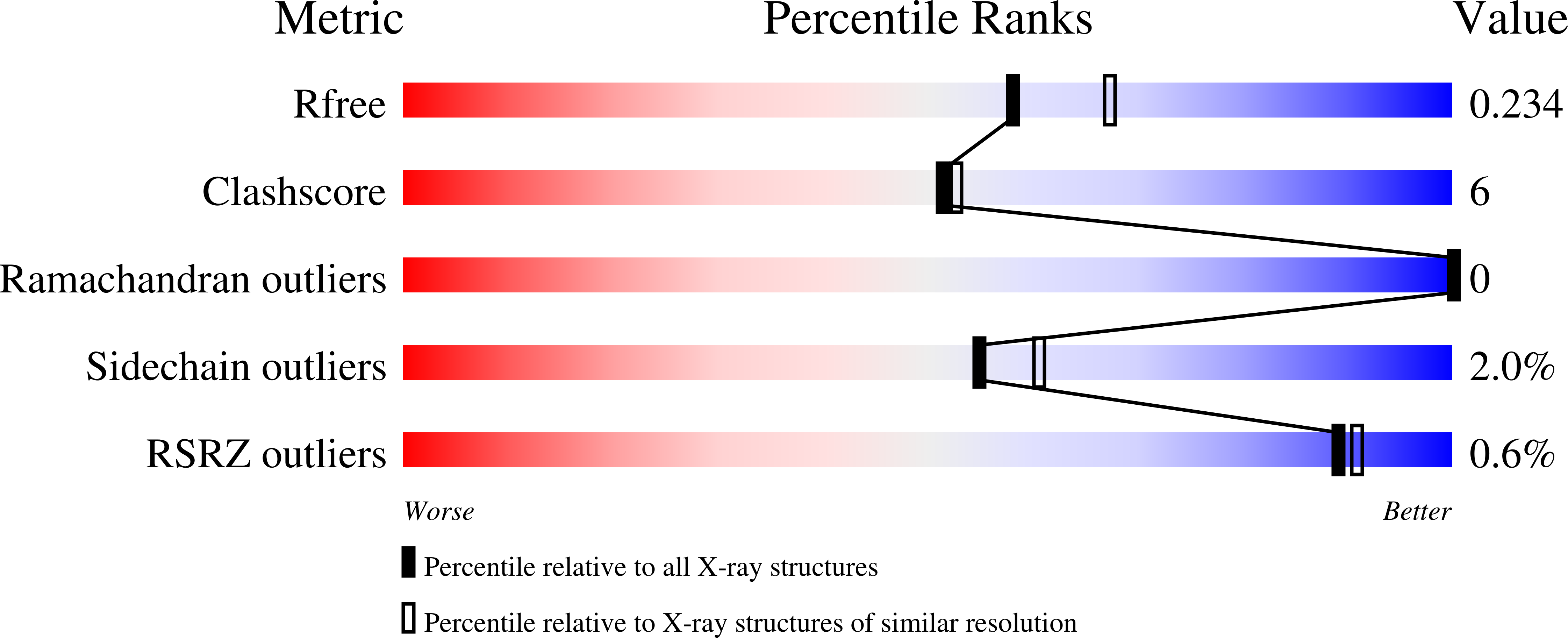
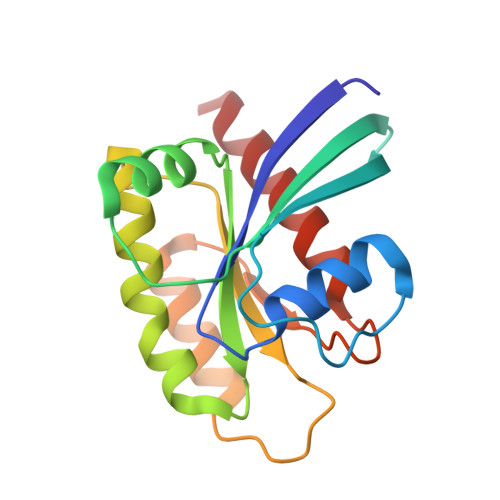
Identification of MRTX1133, a Noncovalent, Potent, and Selective KRAS G12D Inhibitor.
Wang, X., Allen, S., Blake, J.F., Bowcut, V., Briere, D.M., Calinisan, A., Dahlke, J.R., Fell, J.B., Fischer, J.P., Gunn, R.J., Hallin, J., Laguer, J., Lawson, J.D., Medwid, J., Newhouse, B., Nguyen, P., O'Leary, J.M., Olson, P., Pajk, S., Rahbaek, L., Rodriguez, M., Smith, C.R., Tang, T.P., Thomas, N.C., Vanderpool, D., Vigers, G.P., Christensen, J.G., Marx, M.A.(2022) J Med Chem 65: 3123-3133
- PubMed: 34889605
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01688
- Primary Citation of Related Structures:
7RPZ, 7RT1, 7RT2, 7RT3, 7RT4, 7RT5 - PubMed Abstract:
KRAS G12D , the most common oncogenic KRAS mutation, is a promising target for the treatment of solid tumors. However, when compared to KRAS G12C , selective inhibition of KRAS G12D presents a significant challenge due to the requirement of inhibitors to bind KRAS G12D with high enough affinity to obviate the need for covalent interactions with the mutant KRAS protein. Here, we report the discovery and characterization of the first noncovalent, potent, and selective KRAS G12D inhibitor, MRTX1133, which was discovered through an extensive structure-based activity improvement and shown to be efficacious in a KRAS G12D mutant xenograft mouse tumor model.
Organizational Affiliation:
Mirati Therapeutics, San Diego, California 92121, United States.