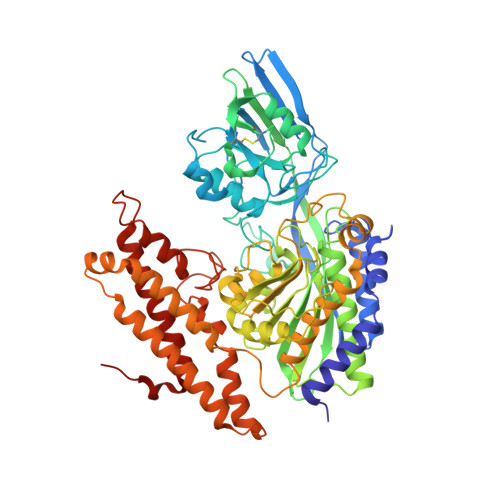
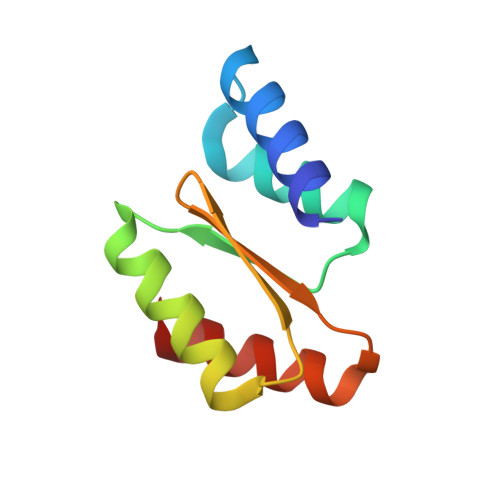
Transferrin receptor targeting by de novo sheet extension.
Sahtoe, D.D., Coscia, A., Mustafaoglu, N., Miller, L.M., Olal, D., Vulovic, I., Yu, T.Y., Goreshnik, I., Lin, Y.R., Clark, L., Busch, F., Stewart, L., Wysocki, V.H., Ingber, D.E., Abraham, J., Baker, D.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 33879614
- DOI: https://doi.org/10.1073/pnas.2021569118
- Primary Citation of Related Structures:
6WRV, 6WRW, 6WRX - PubMed Abstract:
The de novo design of polar protein-protein interactions is challenging because of the thermodynamic cost of stripping water away from the polar groups. Here, we describe a general approach for designing proteins which complement exposed polar backbone groups at the edge of beta sheets with geometrically matched beta strands. We used this approach to computationally design small proteins that bind to an exposed beta sheet on the human transferrin receptor (hTfR), which shuttles interacting proteins across the blood-brain barrier (BBB), opening up avenues for drug delivery into the brain. We describe a design which binds hTfR with a 20 nM K d , is hyperstable, and crosses an in vitro microfluidic organ-on-a-chip model of the human BBB. Our design approach provides a general strategy for creating binders to protein targets with exposed surface beta edge strands.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA 98195.