Use of the 4-Hydroxytriazole Moiety as a Bioisosteric Tool in the Development of Ionotropic Glutamate Receptor Ligands.
Sainas, S., Temperini, P., Farnsworth, J.C., Yi, F., Mollerud, S., Jensen, A.A., Nielsen, B., Passoni, A., Kastrup, J.S., Hansen, K.B., Boschi, D., Pickering, D.S., Clausen, R.P., Lolli, M.L.(2019) J Med Chem 62: 4467-4482
- PubMed: 30943028
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01986
- Primary Citation of Related Structures:
6Q54, 6Q60 - PubMed Abstract:
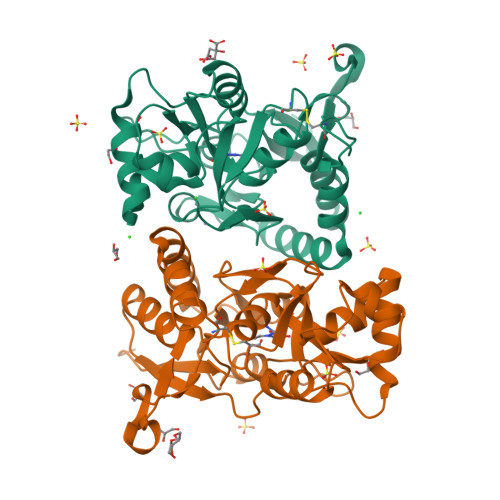
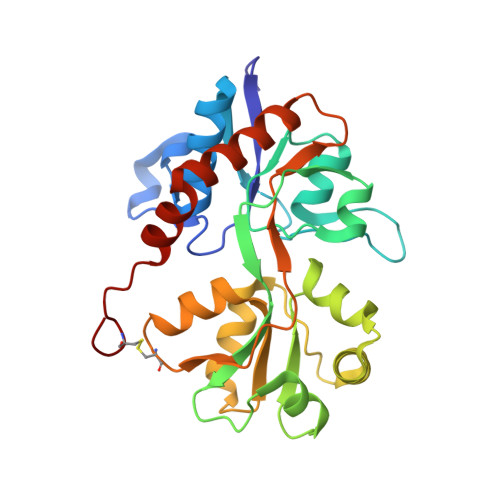
We report a series of glutamate and aspartate analogues designed using the hydroxy-1,2,3-triazole moiety as a bioisostere for the distal carboxylic acid. Compound 6b showed unprecedented selectivity among ( S)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid (AMPA) receptor subtypes, confirmed also by an unusual binding mode observed for the crystal structures in complex with the AMPA receptor GluA2 agonist-binding domain. Here, a methionine (Met729) was highly disordered compared to previous agonist-bound structures. This observation provides a possible explanation for the pharmacological profile. In the structure with 7a, an unusual organization of water molecules around the bioisostere arises compared to previous structures of ligands with other bioisosteres. Aspartate analogue 8 with the hydroxy-1,2,3-triazole moiety directly attached to glycine was unexpectedly able to activate both the glutamate and glycine agonist-binding sites of the N-methyl-d-aspartic acid receptor. These observations demonstrate novel features that arise when employing a hydroxytriazole moiety as a bioisostere for the distal carboxylic acid in glutamate receptor agonists.
Organizational Affiliation:
Department of Drug Science and Technology , University of Turin , via P.Giuria 9 , 10125 Turin , Italy.