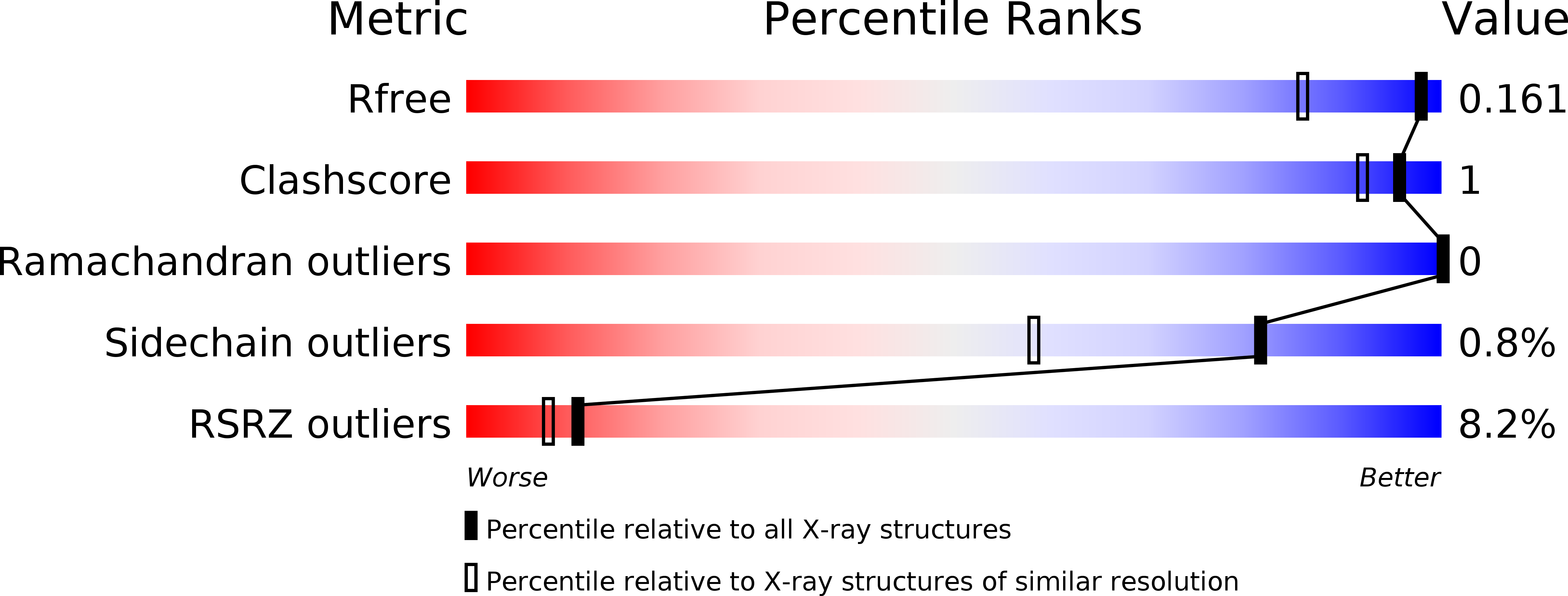
Characterization of 3,3-dimethyl substituted N-aryl piperidines as potent microsomal prostaglandin E synthase-1 inhibitors.
Kuklish, S.L., Antonysamy, S., Bhattachar, S.N., Chandrasekhar, S., Fisher, M.J., Fretland, A.J., Gooding, K., Harvey, A., Hughes, N.E., Luz, J.G., Manninen, P.R., McGee, J.E., Navarro, A., Norman, B.H., Partridge, K.M., Quimby, S.J., Schiffler, M.A., Sloan, A.V., Warshawsky, A.M., York, J.S., Yu, X.P.(2016) Bioorg Med Chem Lett 26: 4824-4828
- PubMed: 27554445
- DOI: https://doi.org/10.1016/j.bmcl.2016.08.023
- Primary Citation of Related Structures:
5K0I - PubMed Abstract:
Here we report on novel, potent 3,3-dimethyl substituted N-aryl piperidine inhibitors of microsomal prostaglandin E synthases-1(mPGES-1). Example 14 potently inhibited PGE2 synthesis in an ex vivo human whole blood (HWB) assay with an IC50 of 7nM. In addition, 14 had no activity in human COX-1 or COX-2 assays at 30μM, and failed to inhibit human mPGES-2 at 62.5μM in a microsomal prep assay. These data are consistent with selective mPGES-1-mediated reduction of PGE2. In dog, 14 had oral bioavailability (74%), clearance (3.62mL/(min*kg)) and volume of distribution (Vd,ss=1.6L/kg) values within our target ranges. For these reasons, 14 was selected for further study.
Organizational Affiliation:
Lilly Research Laboratories, A Division of Eli Lilly and Company, Indianapolis, IN 46285, USA. Electronic address: [email protected].