Triazolopyrimidines identified as reversible myeloperoxidase inhibitors.
Duclos, F., Abell, L.M., Harden, D.G., Pike, K., Nowak, K., Locke, G.A., Duke, G.J., Liu, X., Fernando, G., Shaw, S.A., Vokits, B.P., Wurtz, N.R., Viet, A., Valente, M.N., Stachura, S., Sleph, P., Khan, J.A., Gao, J., Dongre, A.R., Zhao, L., Wexler, R.R., Gordon, D.A., Kick, E.K.(2017) Medchemcomm 8: 2093-2099
- PubMed: 30108726
- DOI: https://doi.org/10.1039/c7md00268h
- Primary Citation of Related Structures:
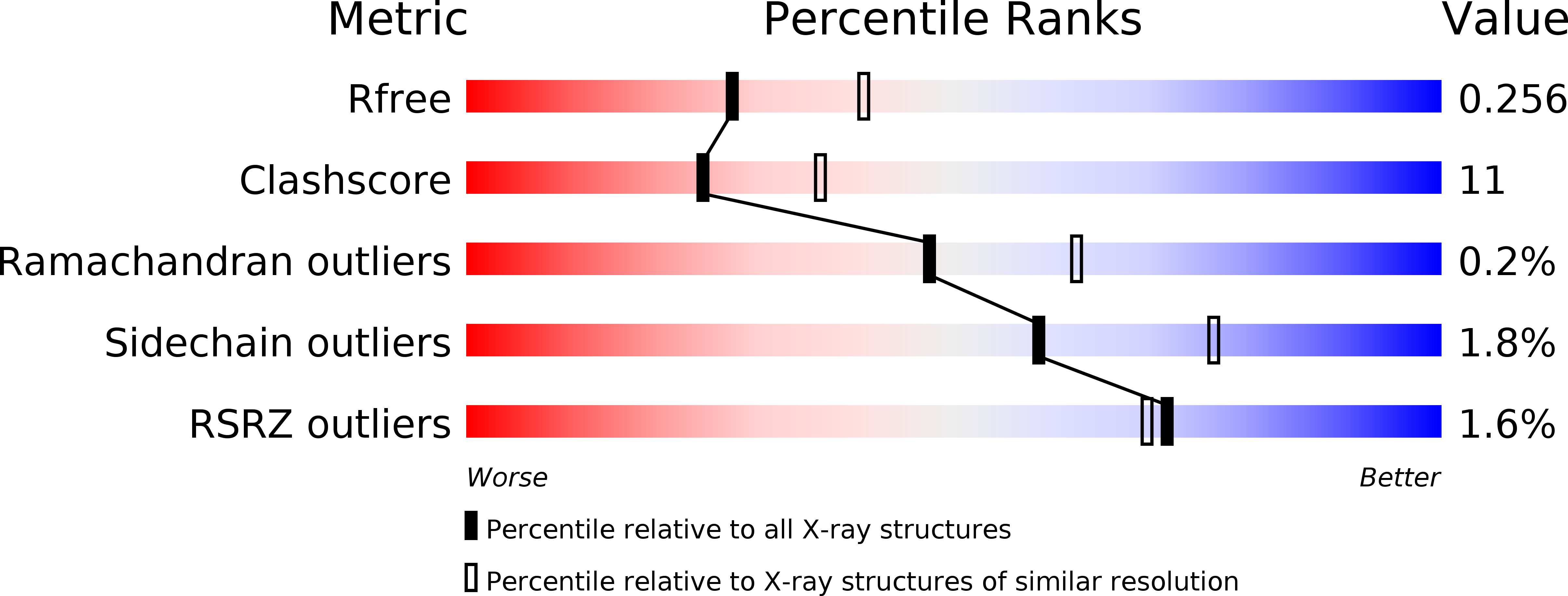
5WDJ - PubMed Abstract:
Myeloperoxidase, a mammalian peroxidase involved in the immune system as an anti-microbial first responder, can produce hypochlorous acid in response to invading pathogens. Myeloperoxidase has been implicated in several chronic pathological diseases due to the chronic production of hypochlorous acid, as well as other reactive radical species. A high throughput screen and triaging protocol was developed to identify a reversible inhibitor of myeloperoxidase toward the potential treatment of chronic diseases such as atherosclerosis. The identification and characterization of a reversible myeloperoxidase inhibitor, 7-(benzyloxy)-3 H -[1,2,3]triazolo[4,5- d ]pyrimidin-5-amine is described.
Organizational Affiliation:
Bristol-Myers Squibb Company , P.O. Box 5400 , Princeton , New Jersey 08543-5400 , USA . Email: [email protected].