Structures of lanosterol 14-alpha demethylase mutants.
Sagatova, A., Keniya, M.V., Wilson, R.K., Sabherwal, M., Tyndall, J.D.A., Monk, B.C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lanosterol 14-alpha demethylase | 539 | Saccharomyces cerevisiae YJM789 | Mutation(s): 1 Gene Names: ERG11, SCY_2394 Membrane Entity: Yes | 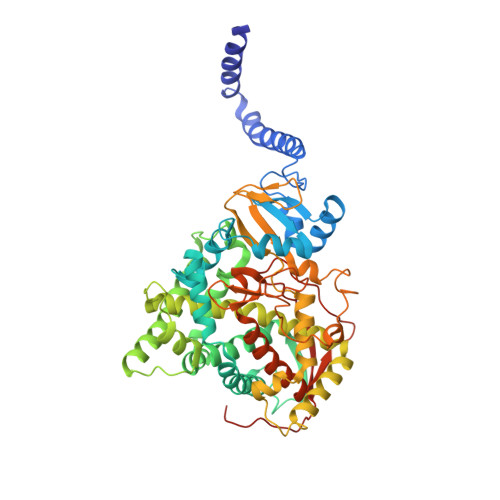 | |
UniProt | |||||
Find proteins for A6ZSR0 (Saccharomyces cerevisiae (strain YJM789)) Explore A6ZSR0 Go to UniProtKB: A6ZSR0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A6ZSR0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 1YN Query on 1YN | B [auth A] | 2-[(2R)-butan-2-yl]-4-{4-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]phenyl}-2,4-dihydro-3H-1,2,4-triazol-3-one C35 H38 Cl2 N8 O4 VHVPQPYKVGDNFY-DFMJLFEVSA-N |  | ||
| HEM Query on HEM | C [auth A] | PROTOPORPHYRIN IX CONTAINING FE C34 H32 Fe N4 O4 KABFMIBPWCXCRK-RGGAHWMASA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 77.09 | α = 90 |
| b = 65.39 | β = 98.42 |
| c = 80.78 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Blu-Ice | data collection |
| Aimless | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Health Research Council (HRC) | New Zealand | -- |
| Marsden Fund | New Zealand | -- |