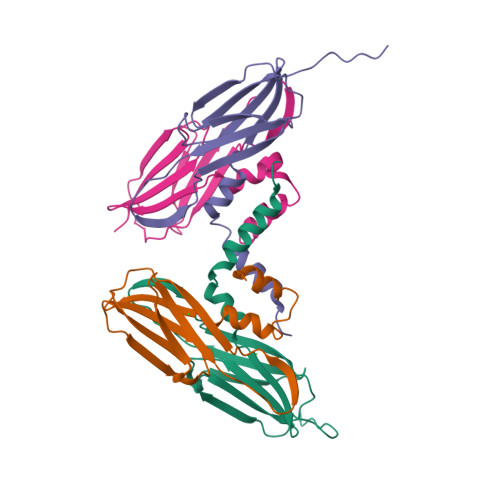
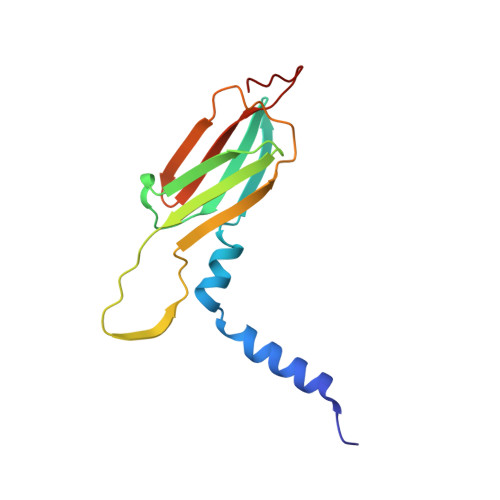
Active-State Structures of a Small Heat-Shock Protein Revealed a Molecular Switch for Chaperone Function
Liu, L., Chen, J.Y., Yang, B., Wang, F.H., Wang, Y.H., Yun, C.H.(2015) Structure 23: 2066-2075
- PubMed: 26439766
- DOI: https://doi.org/10.1016/j.str.2015.08.015
- Primary Citation of Related Structures:
4YL9, 4YLB, 4YLC - PubMed Abstract:
Small heat-shock proteins (sHsps) maintain cellular homeostasis by binding to denatured client proteins to prevent aggregation. Numerous studies indicate that the N-terminal domain (NTD) of sHsps is responsible for binding to client proteins, but the binding mechanism and chaperone activity regulation remain elusive. Here, we report the crystal structures of the wild-type and mutants of an sHsp from Sulfolobus solfataricus representing the inactive and active state of this protein, respectively. All three structures reveal well-defined NTD, but their conformations are remarkably different. The mutant NTDs show disrupted helices presenting a reformed hydrophobic surface compatible with recognizing client proteins. Our functional data show that mutating key hydrophobic residues in this region drastically altered the chaperone activity of this sHsp. These data suggest a new model in which a molecular switch located in NTD facilitates conformational changes for client protein binding.
Organizational Affiliation:
Institute of Systems Biomedicine, Department of Biophysics, Beijing Key Laboratory of Tumor Systems Biology and Center for Molecular and Translational Medicine, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, P.R. China; School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, P.R. China; Co-first author.