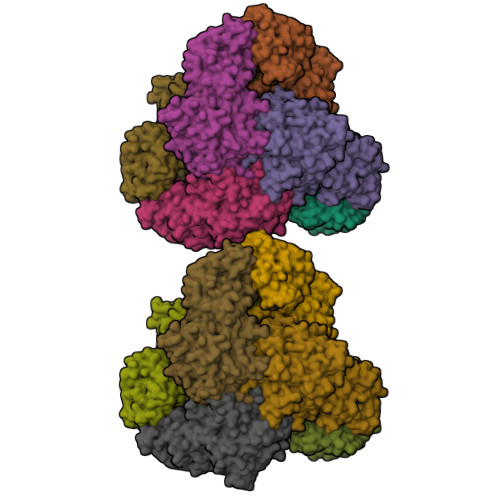
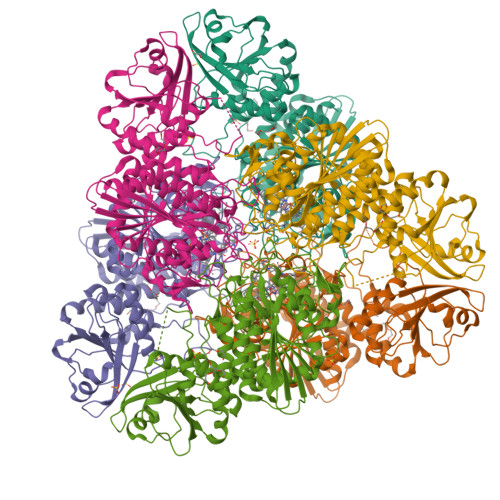
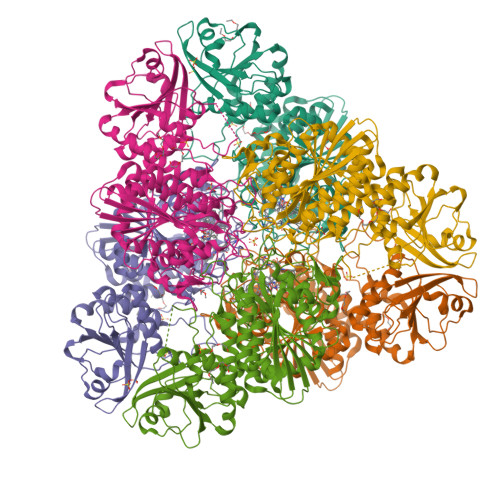
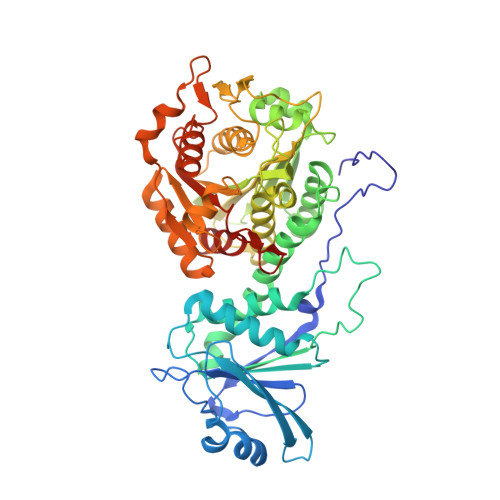
Bestatin-based chemical biology strategy reveals distinct roles for malaria M1- and M17-family aminopeptidases
Harbut, M.B., Velmourougane, G., Dalal, S., Reiss, G., Whisstock, J.C., Onder, O., Brisson, D., McGowan, S., Klemba, M., Greenbaum, D.C.(2011) Proc Natl Acad Sci U S A 108: E526-E534
- PubMed: 21844374
- DOI: https://doi.org/10.1073/pnas.1105601108
- Primary Citation of Related Structures:
3T8V, 3T8W - PubMed Abstract:
Malaria causes worldwide morbidity and mortality, and while chemotherapy remains an excellent means of malaria control, drug-resistant parasites necessitate the discovery of new antimalarials. Peptidases are a promising class of drug targets and perform several important roles during the Plasmodium falciparum erythrocytic life cycle. Herein, we report a multidisciplinary effort combining activity-based protein profiling, biochemical, and peptidomic approaches to functionally analyze two genetically essential P. falciparum metallo-aminopeptidases (MAPs), PfA-M1 and Pf-LAP. Through the synthesis of a suite of activity-based probes (ABPs) based on the general MAP inhibitor scaffold, bestatin, we generated specific ABPs for these two enzymes. Specific inhibition of PfA-M1 caused swelling of the parasite digestive vacuole and prevented proteolysis of hemoglobin (Hb)-derived oligopeptides, likely starving the parasite resulting in death. In contrast, inhibition of Pf-LAP was lethal to parasites early in the life cycle, prior to the onset of Hb degradation suggesting that Pf-LAP has an essential role outside of Hb digestion.
Organizational Affiliation:
Department of Pharmacology, University of Pennsylvania, 433 South University Avenue, 304G Lynch Laboratories, Philadelphia, PA 19104-6018, USA.