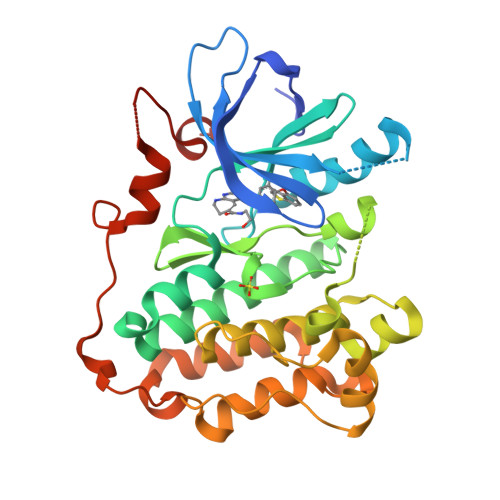
Design and synthesis of novel pyrimido[4,5-b]azepine derivatives as HER2/EGFR dual inhibitors
Kawakita, Y., Seto, M., Ohashi, T., Tamura, T., Yusa, T., Miki, H., Iwata, H., Kamiguchi, H., Tanaka, T., Sogabe, S., Ohta, Y., Ishikawa, T.(2013) Bioorg Med Chem 21: 2250-2261
- PubMed: 23490150
- DOI: https://doi.org/10.1016/j.bmc.2013.02.014
- Primary Citation of Related Structures:
3W32, 3W33 - PubMed Abstract:
A novel 7,6 fused bicyclic scaffold, pyrimido[4,5-b]azepine was designed to fit into the ATP binding site of the HER2/EGFR proteins. The synthesis of this scaffold was accomplished by an intramolecular Claisen-type condensation. As the results of optimization lead us to 4-anilino and 6-functional groups, we discovered 6-substituted amide derivative 19b, which has a 1-benzothiophen-4-yloxy group attached to the 4-anilino group. An X-ray co-crystal structure of 19b with EGFR demonstrated that the N-1 and N-3 nitrogens of the pyrimido[4,5-b]azepine scaffold make hydrogen-bonding interactions with the main chain NH of Met793 and the side chain of Thr854 via a water-mediated hydrogen bond network, respectively. In addition, the NH proton at the 9-position makes an additional hydrogen bond with the carbonyl group of Met793, as we expected. Compound 19b revealed potent HER2/EGFR kinase (IC50: 24/36 nM) and BT474 cell growth (GI50: 18 nM) inhibitory activities based on its pseudo-irreversible (PI) profile.
Organizational Affiliation:
Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd, 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: youichi.kawakita@takeda.com.