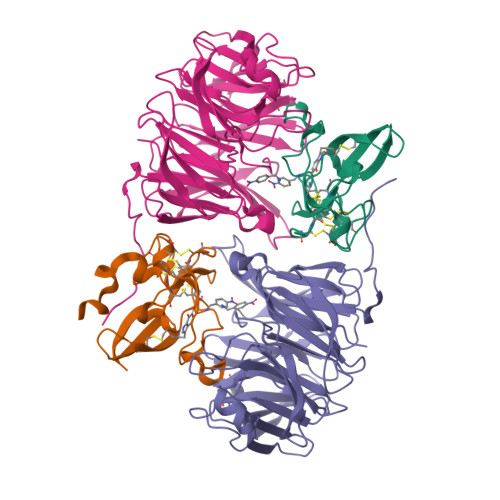
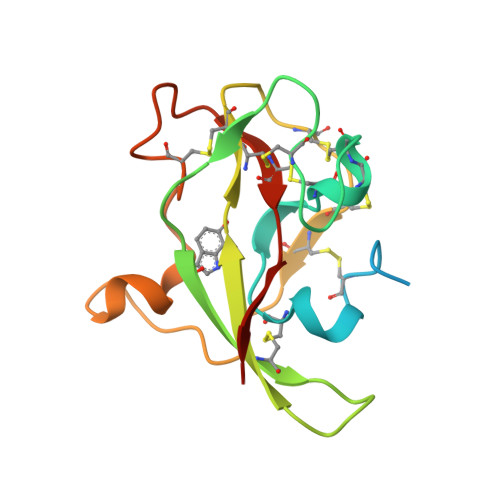
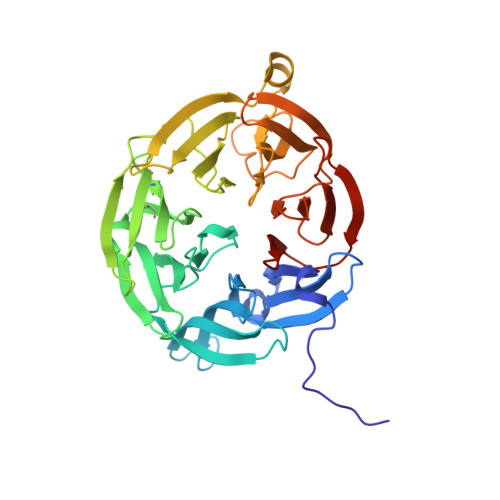
Isotope effects reveal that para-substituted benzylamines are poor reactivity probes of the quinoprotein mechanism for aromatic amine dehydrogenase.
Hothi, P., Roujeinikova, A., Khadra, K.A., Lee, M., Cullis, P., Leys, D., Scrutton, N.S.(2007) Biochemistry 46: 9250-9259
- PubMed: 17636875
- DOI: https://doi.org/10.1021/bi7007239
- Primary Citation of Related Structures:
2HJ4, 2HJB, 2Q7Q - PubMed Abstract:
Structure-activity correlations have been employed previously in the mechanistic interpretation of TTQ-dependent amine dehydrogenases using a series of para-substituted benzylamines. However, by combining the use of kinetic isotope effects (KIEs) and crystallographic analysis, in conjunction with structure-reactivity correlation studies, we show that para-substituted benzylamines are poor reactivity probes for TTQ-dependent aromatic amine dehydrogenase (AADH). Stopped-flow kinetic studies of the reductive half-reaction, with para-substituted benzylamines and their dideuterated counterparts, demonstrate that C-H or C-D bond breakage is not fully rate limiting (KIEs approximately unity). Contrary to previous reports, Hammett plots exhibit a poor correlation of structure-reactivity data with electronic substituent effects for para-substituted benzylamines and phenylethylamines. Crystallographic studies of enzyme-substrate complexes reveal that the observed structure-reactivity correlations are not attributed to distinct binding modes for para-substituted benzylamines in the active site, although two binding sites for p-nitrobenzylamine are identified. We identify structural rearrangements, prior to the H-transfer step, which are likely to limit the rate of TTQ reduction by benzylamines. This work emphasizes (i) the need for caution when applying structure-activity correlations to enzyme-catalyzed reactions and (ii) the added benefit of using both isotope effects and structural analysis, in conjunction with structure-reactivity relationships, to study chemical steps in enzyme reaction cycles.
Organizational Affiliation:
Manchester Interdisciplinary Biocentre, Faculty of Life Sciences, University of Manchester, UK.