Crystal Structure and Biochemical Properties of the d-Arabinose Dehydrogenase from Sulfolobus solfataricus
Brouns, S.J., Turnbull, A.P., Willemen, H.L., Akerboom, J., van der Oost, J.(2007) J Mol Biol 371: 1249-1260
- PubMed: 17610898
- DOI: https://doi.org/10.1016/j.jmb.2007.05.097
- Primary Citation of Related Structures:
2H6E - PubMed Abstract:
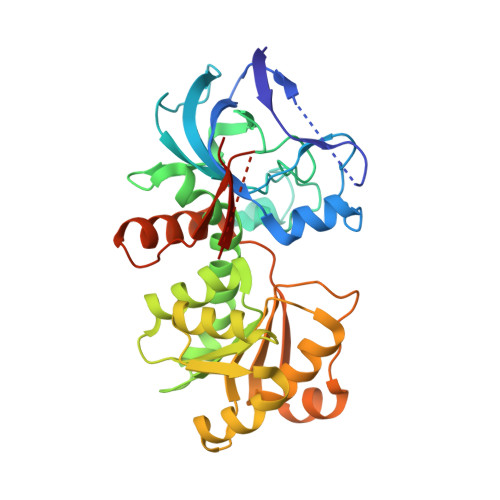
Sulfolobus solfataricus metabolizes the five-carbon sugar d-arabinose to 2-oxoglutarate by an inducible pathway consisting of dehydrogenases and dehydratases. Here we report the crystal structure and biochemical properties of the first enzyme of this pathway: the d-arabinose dehydrogenase. The AraDH structure was solved to a resolution of 1.80 A by single-wavelength anomalous diffraction and phased using the two endogenous zinc ions per subunit. The structure revealed a catalytic and cofactor binding domain, typically present in mesophilic and thermophilic alcohol dehydrogenases. Cofactor modeling showed the presence of a phosphate binding pocket sequence motif (SRS-X2-H), which is likely to be responsible for the enzyme's preference for NADP+. The homo-tetrameric enzyme is specific for d-arabinose, l-fucose, l-galactose and d-ribose, which could be explained by the hydrogen bonding patterns of the C3 and C4 hydroxyl groups observed in substrate docking simulations. The enzyme optimally converts sugars at pH 8.2 and 91 degrees C, and displays a half-life of 42 and 26 min at 85 and 90 degrees C, respectively, indicating that the enzyme is thermostable at physiological operating temperatures of 80 degrees C. The structure represents the first crystal structure of an NADP+-dependent member of the medium-chain dehydrogenase/reductase (MDR) superfamily from Archaea.
Organizational Affiliation:
Laboratory of Microbiology, Department of Agrotechnology and Food Sciences, Wageningen University, Hesselink van Suchtelenweg 4, 6703 CT Wageningen, Netherlands. [email protected]