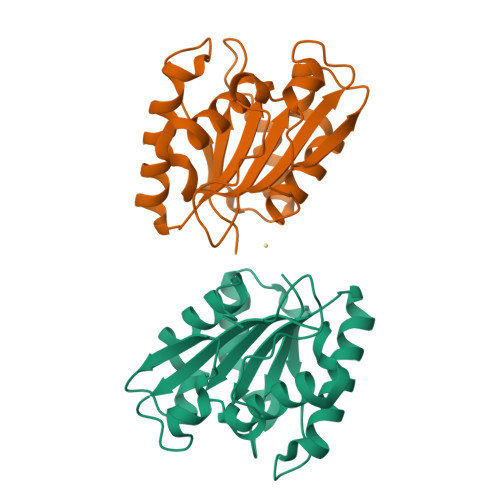
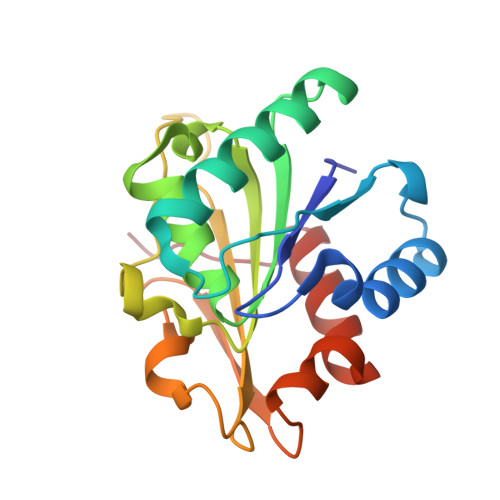
The crystal structure of Bacillus subtilis lipase: a minimal alpha/beta hydrolase fold enzyme.
van Pouderoyen, G., Eggert, T., Jaeger, K.E., Dijkstra, B.W.(2001) J Mol Biology 309: 215-226
- PubMed: 11491291
- DOI: https://doi.org/10.1006/jmbi.2001.4659
- Primary Citation of Related Structures:
1I6W - PubMed Abstract:
The X-ray structure of the lipase LipA from Bacillus subtilis has been determined at 1.5 A resolution. It is the first structure of a member of homology family 1.4 of bacterial lipases. The lipase shows a compact minimal alpha/beta hydrolase fold with a six-stranded parallel beta-sheet flanked by five alpha-helices, two on one side of the sheet and three on the other side. The catalytic triad residues, Ser77, Asp133 and His156, and the residues forming the oxyanion hole (backbone amide groups of Ile12 and Met78) are in positions very similar to those of other lipases of known structure. However, no lid domain is present and the active-site nucleophile Ser77 is solvent-exposed. A model of substrate binding is proposed on the basis of a comparison with other lipases with a covalently bound tetrahedral intermediate mimic. It explains the preference of the enzyme for substrates with C8 fatty acid chains.
Organizational Affiliation:
Laboratory of Biophysical Chemistry, University of Groningen, The Netherlands.