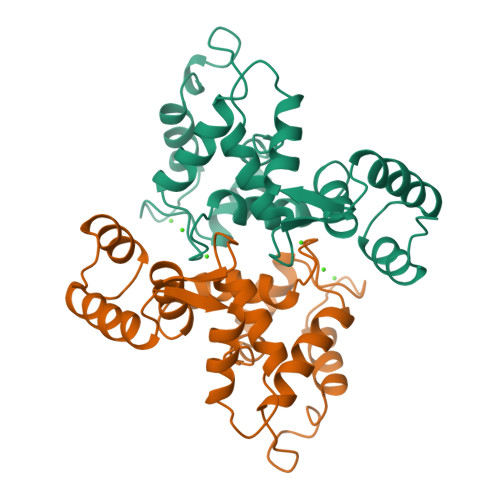
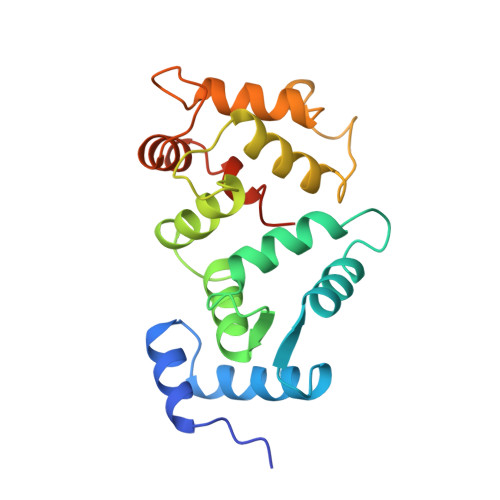
Crystal structure of recombinant bovine neurocalcin.
Vijay-Kumar, S., Kumar, V.D.(1999) Nat Struct Biol 6: 80-88
- PubMed: 9886296
- DOI: https://doi.org/10.1038/4956
- Primary Citation of Related Structures:
1BJF - PubMed Abstract:
The crystal structure of calcium-bound unmyristoylated bovine neurocalcin from Escherichia coli has been determined at 2.4 A resolution. The three-dimensional structure reveals a highly compact structure consisting of: (i) two pairs of calcium-binding EF-hands (EF1-EF2 and EF3-EF4); (ii) a calcium ion bound at EF2, EF3 and EF4 sites; and (iii) an EF1-hand that is disabled from calcium-binding due to a Cys-Pro sequence in the Ca2+-binding loop. The crystal structure of neurocalcin resembles photoreceptor recoverin in overall topology, however its EF2- and EF4-hands differ. Recently, neurocalcin in the calcium-bound state has been shown to stimulate mammalian rod outer segment membrane guanylate cyclase. A possible site for cyclase activity based on the three-dimensional structure is discussed.
Organizational Affiliation:
Department of Biochemistry, Fels Institute for Cancer Research and Molecular Biology, Temple University School of Medicine, Philadelphia, Pennsylvania 19140, USA.