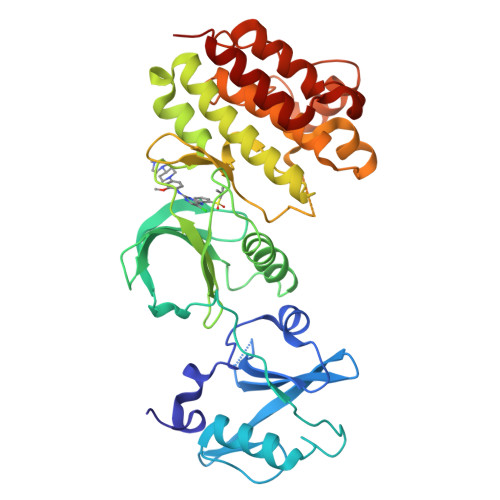
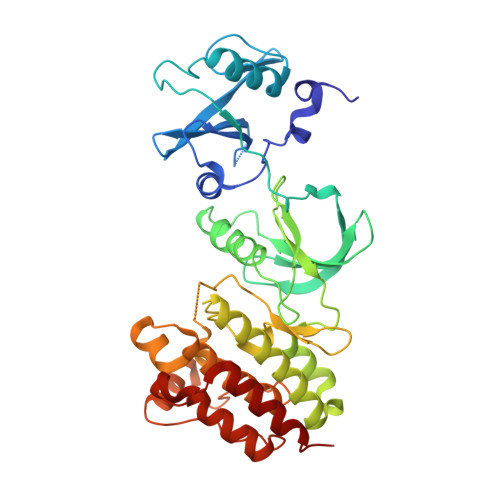
Small-Molecule Inhibitors of the c-Fes Protein-Tyrosine Kinase.
Hellwig, S., Miduturu, C.V., Kanda, S., Zhang, J., Filippakopoulos, P., Salah, E., Deng, X., Choi, H.G., Zhou, W., Hur, W., Knapp, S., Gray, N.S., Smithgall, T.E.(2012) Chem Biol 19: 529-540
- PubMed: 22520759
- DOI: https://doi.org/10.1016/j.chembiol.2012.01.020
- Primary Citation of Related Structures:
4E93 - PubMed Abstract:
The c-Fes protein-tyrosine kinase modulates cellular signaling pathways governing differentiation, the innate immune response, and vasculogenesis. Here, we report the identification of types I and II kinase inhibitors with potent activity against c-Fes both in vitro and in cell-based assays. One of the most potent inhibitors is the previously described anaplastic lymphoma kinase inhibitor TAE684. The crystal structure of TAE684 in complex with the c-Fes SH2-kinase domain showed excellent shape complementarity with the ATP-binding pocket and a key role for the gatekeeper methionine in the inhibitory mechanism. TAE684 and two pyrazolopyrimidines with nanomolar potency against c-Fes in vitro were used to establish a role for this kinase in osteoclastogenesis, illustrating the value of these inhibitors as tool compounds to probe the diverse biological functions associated with this unique kinase.
Organizational Affiliation:
Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, Pittsburgh, PA 15219, USA.