Potent pyrimidinetrione-based inhibitors of MMP-13 with enhanced selectivity over MMP-14.
Blagg, J.A., Noe, M.C., Wolf-Gouveia, L.A., Reiter, L.A., Laird, E.R., Chang, S.P., Danley, D.E., Downs, J.T., Elliott, N.C., Eskra, J.D., Griffiths, R.J., Hardink, J.R., Haugeto, A.I., Jones, C.S., Liras, J.L., Lopresti-Morrow, L.L., Mitchell, P.G., Pandit, J., Robinson, R.P., Subramanyam, C., Vaughn-Bowser, M.L., Yocum, S.A.(2005) Bioorg Med Chem Lett 15: 1807-1810
- PubMed: 15780611
- DOI: https://doi.org/10.1016/j.bmcl.2005.02.038
- Primary Citation of Related Structures:
1YOU - PubMed Abstract:
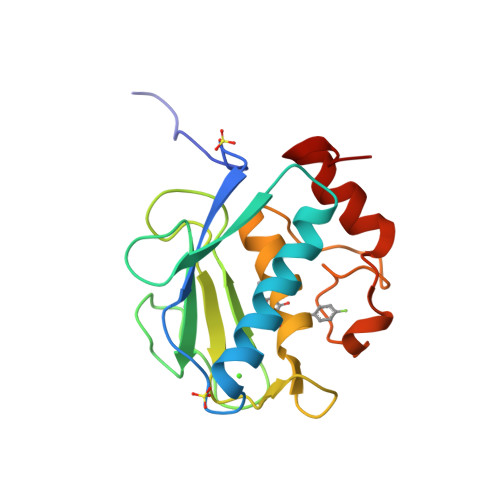
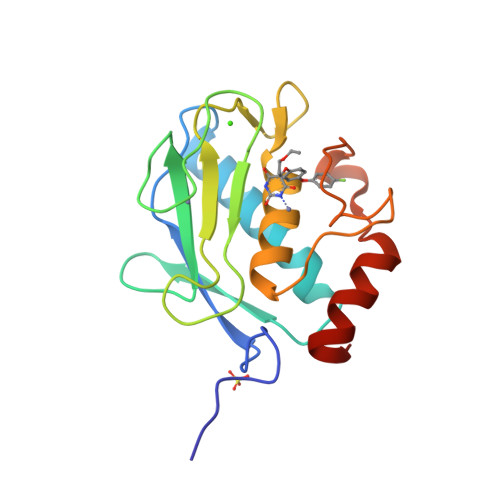
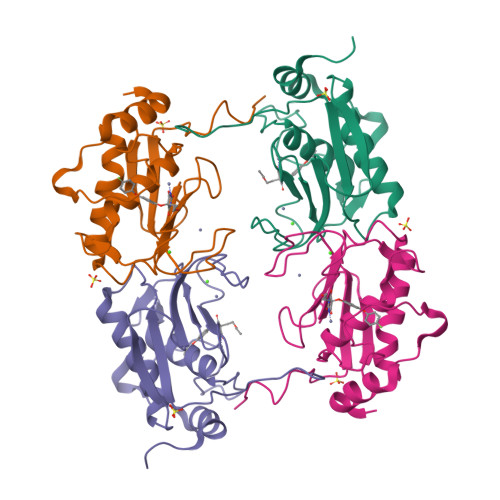
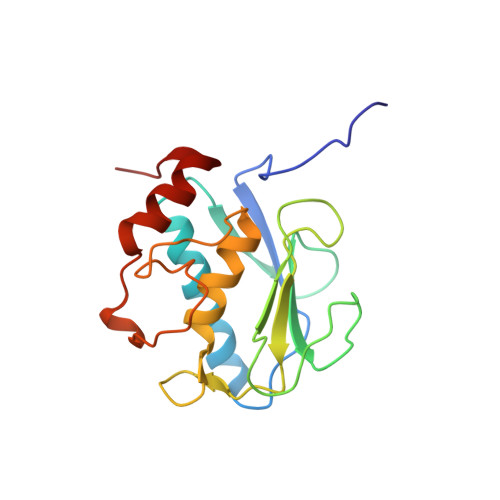
Through the use of computational modeling, a series of pyrimidinetrione-based inhibitors of MMP-13 was designed based on a lead inhibitor identified through file screening. Incorporation of a biaryl ether moiety at the C-5 position of the pyrimidinetrione ring resulted in a dramatic enhancement of MMP-13 potency. Protein crystallography revealed that this moiety binds in the S(1)(') pocket of the enzyme. Optimization of the C-4 substituent of the terminal aromatic ring led to incorporation of selectivity versus MMP-14 (MT-1 MMP). Structure activity relationships of the biaryl ether substituent are presented as is pharmacokinetic data for a compound that meets our in vitro potency and selectivity goals.
Organizational Affiliation:
Pfizer Global Research and Development, Groton Laboratories, MS8220-2471, Eastern Point Road, Groton, CT 06340, USA.