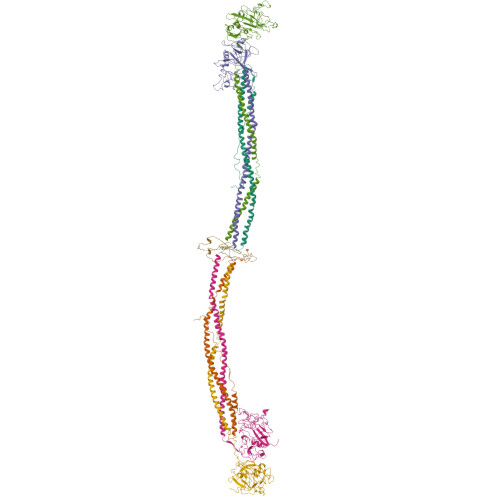
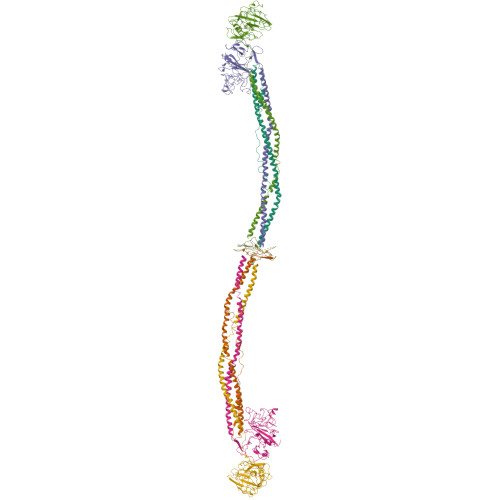
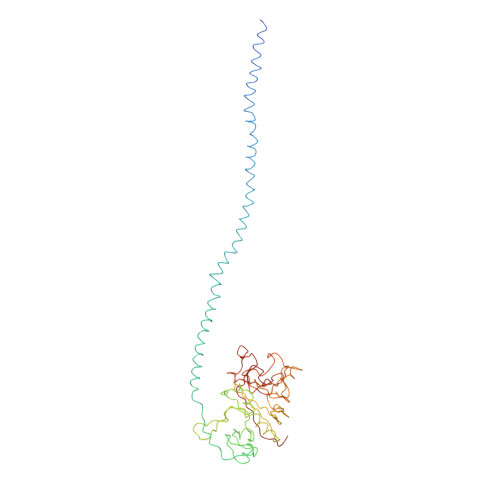
The crystal structure of modified bovine fibrinogen.
Brown, J.H., Volkmann, N., Jun, G., Henschen-Edman, A.H., Cohen, C.(2000) Proc Natl Acad Sci U S A 97: 85-90
- PubMed: 10618375
- DOI: https://doi.org/10.1073/pnas.97.1.85
- Primary Citation of Related Structures:
1DEQ - PubMed Abstract:
Here we report the crystal structure at approximately 4-A resolution of a selectively proteolyzed bovine fibrinogen. This key component in hemostasis is an elongated 340-kDa glycoprotein in the plasma that upon activation by thrombin self-assembles to form the fibrin clot. The crystals are unusual because they are made up of end-to-end bonded molecules that form flexible filaments. We have visualized the entire coiled-coil region of the molecule, which has a planar sigmoidal shape. The primary polymerization receptor pockets at the ends of the molecule face the same way throughout the end-to-end bonded filaments, and based on this conformation, we have developed an improved model of the two-stranded protofibril that is the basic building block in fibrin. Near the middle of the coiled-coil region, the plasmin-sensitive segment is a hinge about which the molecule adopts different conformations. This segment also includes the boundary between the three- and four-stranded portions of the coiled coil, indicating the location on the backbone that anchors the extended flexible Aalpha arm. We suggest that a flexible branch point in the molecule may help accommodate variability in the structure of the fibrin clot.
Organizational Affiliation:
Rosenstiel Basic Medical Sciences Research Center, Brandeis University, Waltham, MA 02454-9110, USA.