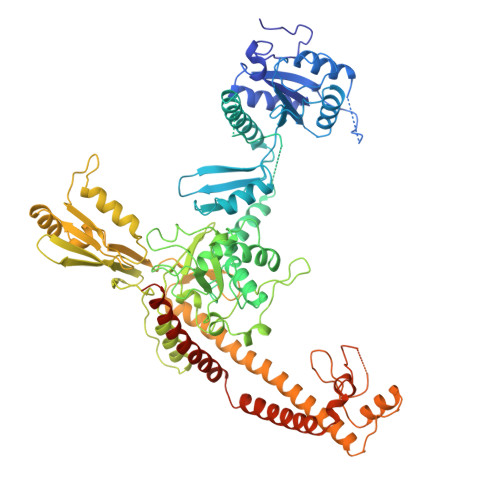
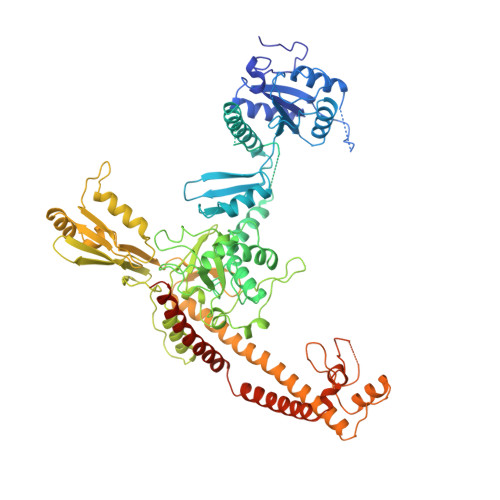
Quaternary changes in topoisomerase II may direct orthogonal movement of two DNA strands.
Fass, D., Bogden, C.E., Berger, J.M.(1999) Nat Struct Biol 6: 322-326
- PubMed: 10201398
- DOI: https://doi.org/10.1038/7556
- Primary Citation of Related Structures:
1BJT - PubMed Abstract:
Type II DNA topoisomerases mediate the passage of one DNA duplex through a transient break in another, an event essential for chromosome segregation and cell viability. The active sites of the type II topoisomerase dimer associate covalently with the DNA break-points and must separate by at least the width of the second DNA duplex to accommodate transport. A new structure of the Saccharomyces cerevisiae topoisomerase II DNA-binding and cleavage core suggests that in addition to conformational changes in the DNA-opening platform, a dramatic reorganization of accessory domains may occur during catalysis. These conformational differences have implications for both the DNA-breaking and duplex-transport events in the topo II reaction mechanism, suggest a mechanism by which two distinct drug-resistance loci interact, and illustrate the scope of structural changes in the cycling of molecular machines.
Organizational Affiliation:
Whitehead Institute for Biomedical Research, Cambridge, Massachusetts 02140, USA.