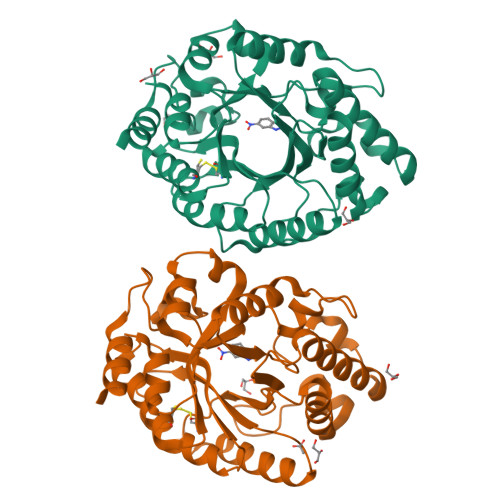
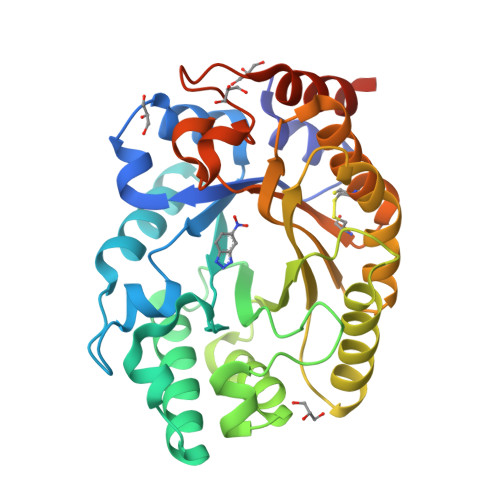
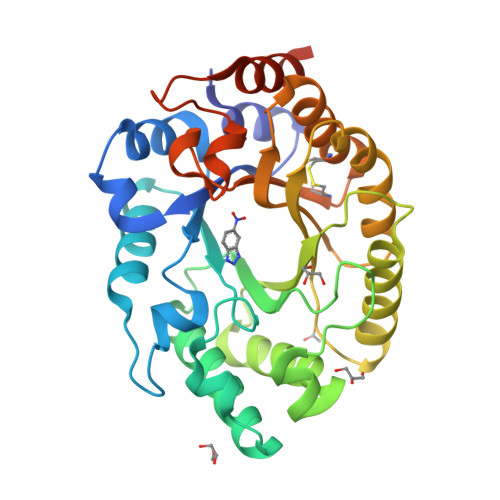
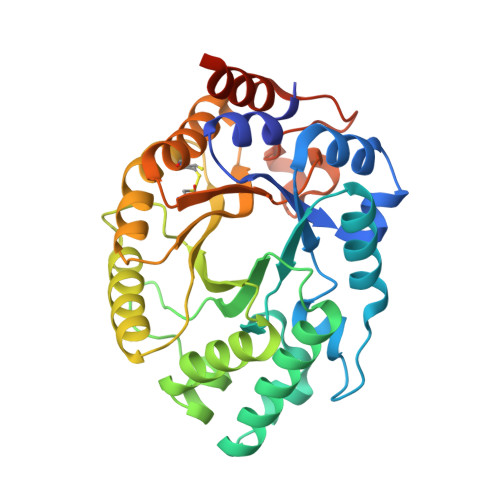
Enriching productive mutational paths accelerates enzyme evolution.
Patsch, D., Schwander, T., Voss, M., Schaub, D., Huppi, S., Eichenberger, M., Stockinger, P., Schelbert, L., Giger, S., Peccati, F., Jimenez-Oses, G., Mutny, M., Krause, A., Bornscheuer, U.T., Hilvert, D., Buller, R.M.(2024) Nat Chem Biol 20: 1662-1669
- PubMed: 39261644
- DOI: https://doi.org/10.1038/s41589-024-01712-3
- Primary Citation of Related Structures:
8RD5 - PubMed Abstract:
Darwinian evolution has given rise to all the enzymes that enable life on Earth. Mimicking natural selection, scientists have learned to tailor these biocatalysts through recursive cycles of mutation, selection and amplification, often relying on screening large protein libraries to productively modulate the complex interplay between protein structure, dynamics and function. Here we show that by removing destabilizing mutations at the library design stage and taking advantage of recent advances in gene synthesis, we can accelerate the evolution of a computationally designed enzyme. In only five rounds of evolution, we generated a Kemp eliminase-an enzymatic model system for proton transfer from carbon-that accelerates the proton abstraction step >10 8 -fold over the uncatalyzed reaction. Recombining the resulting variant with a previously evolved Kemp eliminase HG3.17, which exhibits similar activity but differs by 29 substitutions, allowed us to chart the topography of the designer enzyme's fitness landscape, highlighting that a given protein scaffold can accommodate several, equally viable solutions to a specific catalytic problem.
Organizational Affiliation:
Competence Center for Biocatalysis, Zurich University of Applied Sciences, Waedenswil, Switzerland.