Exploring thienothiadiazine dioxides as isosteric analogues of benzo- and pyridothiadiazine dioxides in the search of new AMPA and kainate receptor positive allosteric modulators.
Francotte, P., Bay, Y., Goffin, E., Colson, T., Lesenfants, C., Dorosz, J., Laulumaa, S., Fraikin, P., de Tullio, P., Beaufour, C., Botez, I., Pickering, D.S., Frydenvang, K., Danober, L., Kristensen, A.S., Kastrup, J.S., Pirotte, B.(2023) Eur J Med Chem 264: 116036-116036
- PubMed: 38101041
- DOI: https://doi.org/10.1016/j.ejmech.2023.116036
- Primary Citation of Related Structures:
8QEZ - PubMed Abstract:
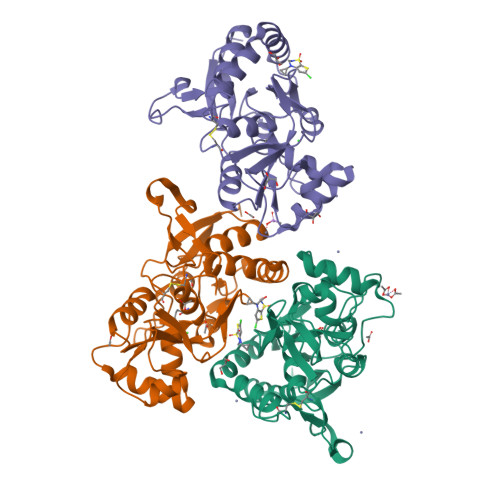
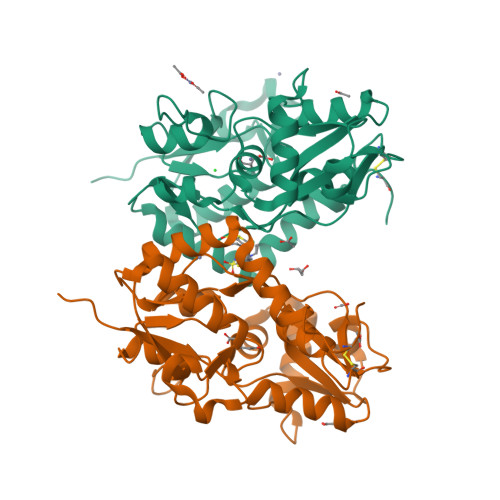
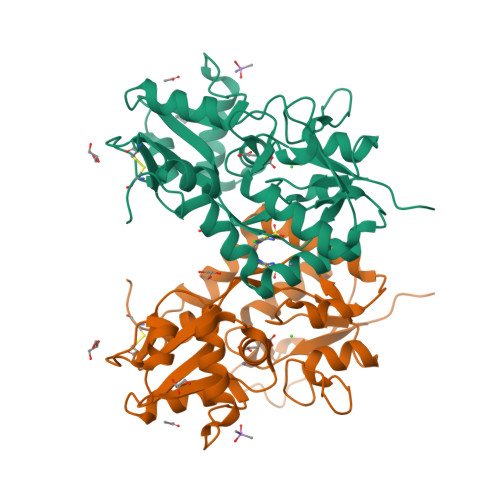
The synthesis and biological evaluation on AMPA and kainate receptors of new examples of 3,4-dihydro-2H-1,2,4-thieno[3,2-e]-1,2,4-thiadiazine 1,1-dioxides is described. The introduction of a cyclopropyl chain instead of an ethyl chain at the 4-position of the thiadiazine ring was found to dramatically improve the potentiator activity on AMPA receptors, with compound 32 (BPAM395) expressing in vitro activity on AMPARs (EC2x = 0.24 μM) close to that of the reference 4-cyclopropyl-substituted benzothiadiazine dioxide 10 (BPAM344). Interestingly, the 4-allyl-substituted thienothiadiazine dioxide 27 (BPAM307) emerged as the most promising compound on kainate receptors being a more effective potentiator than the 4-cyclopropyl-substituted thienothiadiazine dioxide 32 and supporting the view that the 4-allyl substitution of the thiadiazine ring could be more favorable than the 4-cyclopropyl substitution to induce marked activity on kainate receptors versus AMPA receptors. The thieno-analogue 36 (BPAM279) of the clinically tested S18986 (11) was selected for in vivo evaluation in mice as a cognitive enhancer due to a safer profile than 32 after massive per os drug administration. Compound 36 was found to increase the cognition performance in mice at low doses (1 mg/kg) per os suggesting that the compound was well absorbed after oral administration and able to reach the central nervous system. Finally, compound 32 was selected for co-crystallization with the GluA2-LBD (L504Y,N775S) and glutamate to examine the binding mode of thienothiadiazine dioxides within the allosteric binding site of the AMPA receptor. At the allosteric site, this compound established similar interactions as the previously reported BTD-type AMPA receptor modulators.
Organizational Affiliation:
Center for Interdisciplinary Research on Medicines (CIRM) - Laboratory of Medicinal Chemistry, University of Liège, Avenue Hippocrate 15 (B36), B-4000, Liège, Belgium.