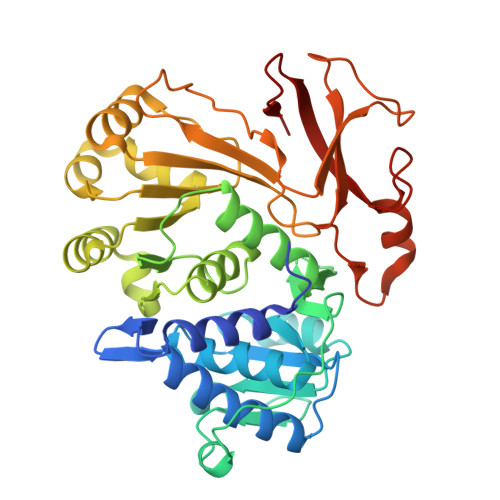
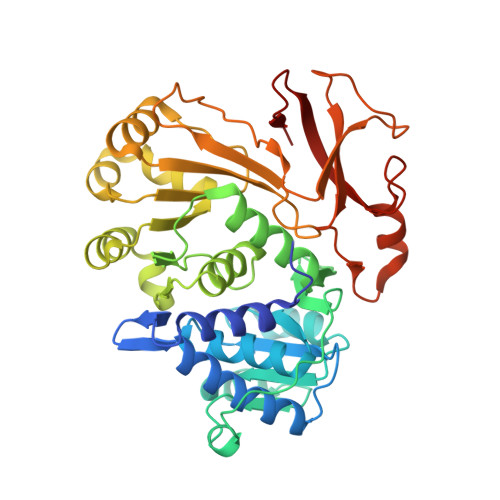
Directed Evolution of Piperazic Acid Incorporation by a Nonribosomal Peptide Synthetase.
Stephan, P., Langley, C., Winkler, D., Basquin, J., Caputi, L., O'Connor, S.E., Kries, H.(2023) Angew Chem Int Ed Engl 62: e202304843-e202304843
- PubMed: 37326625
- DOI: https://doi.org/10.1002/anie.202304843
- Primary Citation of Related Structures:
8P5O - PubMed Abstract:
Engineering of biosynthetic enzymes is increasingly employed to synthesize structural analogues of antibiotics. Of special interest are nonribosomal peptide synthetases (NRPSs) responsible for the production of important antimicrobial peptides. Here, directed evolution of an adenylation domain of a Pro-specific NRPS module completely switched substrate specificity to the non-standard amino acid piperazic acid (Piz) bearing a labile N-N bond. This success was achieved by UPLC-MS/MS-based screening of small, rationally designed mutant libraries and can presumably be replicated with a larger number of substrates and NRPS modules. The evolved NRPS produces a Piz-derived gramicidin S analogue. Thus, we give new impetus to the too-early dismissed idea that widely accessible low-throughput methods can switch the specificity of NRPSs in a biosynthetically useful fashion.
Organizational Affiliation:
Junior Research Group Biosynthetic Design of Natural Products, Leibniz Institute for Natural Product Research and Infection Biology (HKI), Beutenbergstr. 11a, 07745, Jena, Germany.