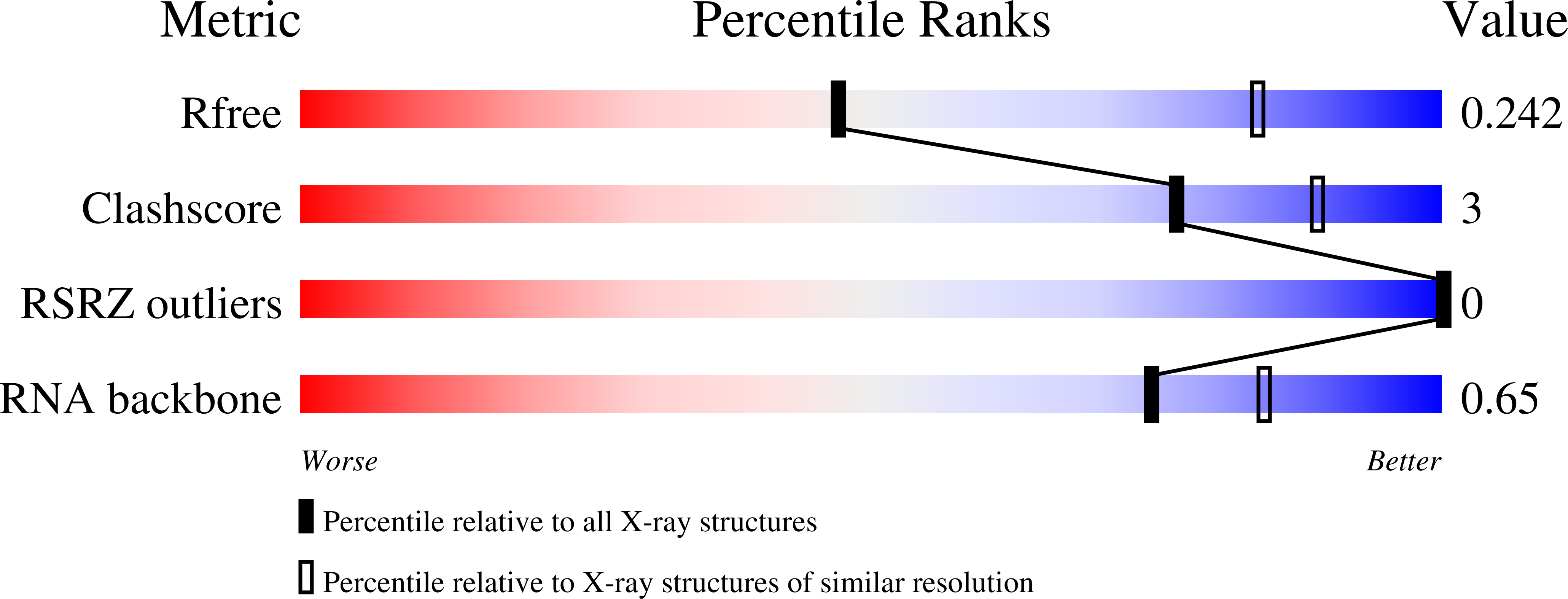The fluorescent aptamer Squash extensively repurposes the adenine riboswitch fold.
Truong, L., Kooshapur, H., Dey, S.K., Li, X., Tjandra, N., Jaffrey, S.R., Ferre-D'Amare, A.R.(2022) Nat Chem Biol 18: 191-198
- PubMed: 34937911
- DOI: https://doi.org/10.1038/s41589-021-00931-2
- Primary Citation of Related Structures:
7KVT, 7KVU, 7KVV - PubMed Abstract:
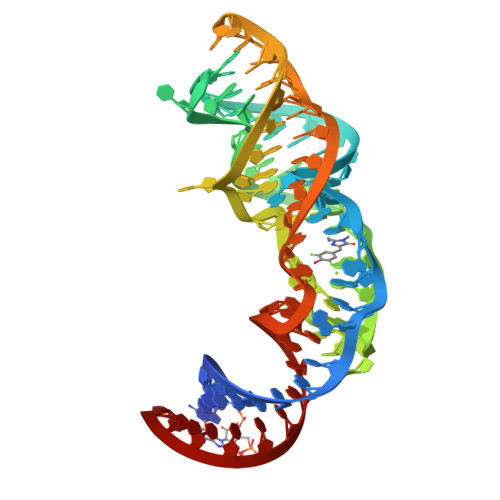
Squash is an RNA aptamer that strongly activates the fluorescence of small-molecule analogs of the fluorophore of green fluorescent protein (GFP). Unlike other fluorogenic aptamers, isolated de novo from random-sequence RNA, Squash was evolved from the bacterial adenine riboswitch to leverage its optimized in vivo folding and stability. We now report the 2.7-Å resolution cocrystal structure of fluorophore-bound Squash, revealing that while the overall fold of the riboswitch is preserved, the architecture of the ligand-binding core is dramatically transformed. Unlike previously characterized aptamers that activate GFP-derived fluorophores, Squash does not harbor a G-quadruplex, sandwiching its fluorophore between a base triple and a noncanonical base quadruple in a largely apolar pocket. The expanded structural core of Squash allows it to recognize unnatural fluorophores that are larger than the simple purine ligand of the parental adenine riboswitch, and suggests that stable RNA scaffolds can tolerate larger variation than has hitherto been appreciated.
Organizational Affiliation:
Biochemistry and Biophysics Center, National Heart, Lung, and Blood Institute, Bethesda, MD, USA.