Discovery of High Affinity Inhibitors of Leishmania Donovani N-Myristoyltransferase.
Rackham, M.D., Yu, Z., Brannigan, J.A., Heal, W.P., Paape, D., Barker, K.V., Wilkinson, A.J., Smith, D.F., Leatherbarrow, R.J., Tate, E.W.(2015) Medchemcomm 6: 1761
- PubMed: 26962429
- DOI: https://doi.org/10.1039/c5md00241a
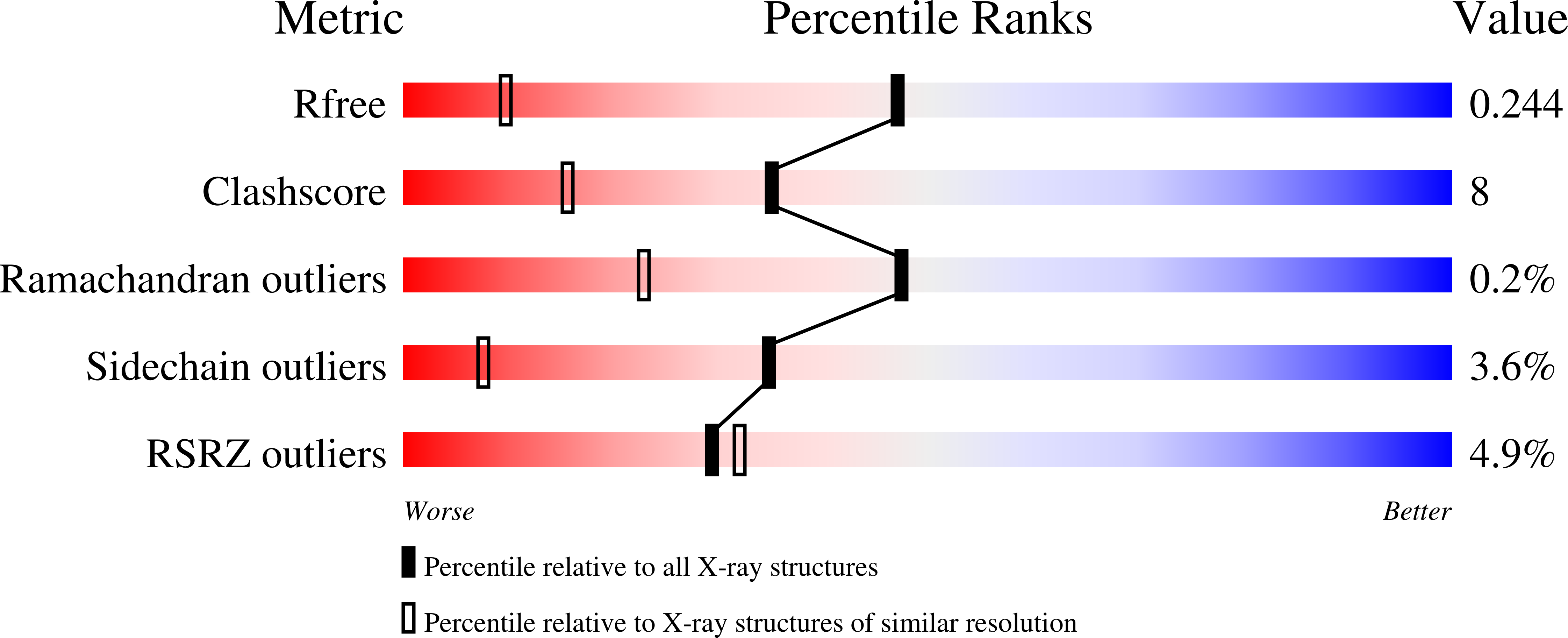
- Primary Citation of Related Structures:
5A27, 5A28 - PubMed Abstract:
N -Myristoyltransferase (NMT) is a potential drug target in Leishmania parasites. Scaffold-hopping from published inhibitors yielded the serendipitous discovery of a chemotype selective for Leishmania donovani NMT; development led to high affinity inhibitors with excellent ligand efficiency. The binding mode was characterised by crystallography and provides a structural rationale for selectivity.
Organizational Affiliation:
Department of Chemistry , Imperial College London , South Kensington Campus , London , SW7 2AZ , UK . Email: [email protected] ; Tel: +44 (0) 2075 943752.