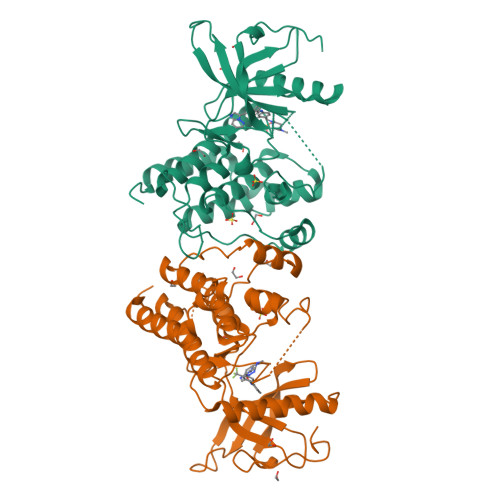
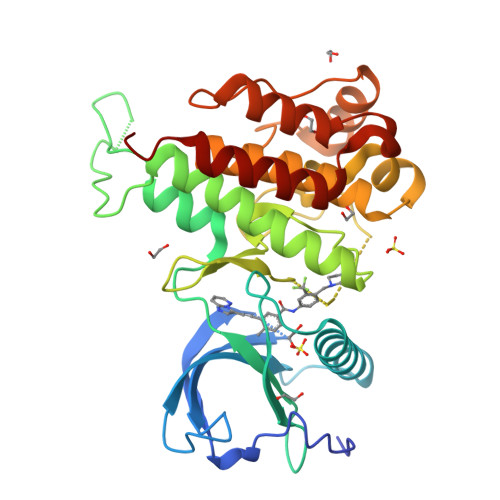
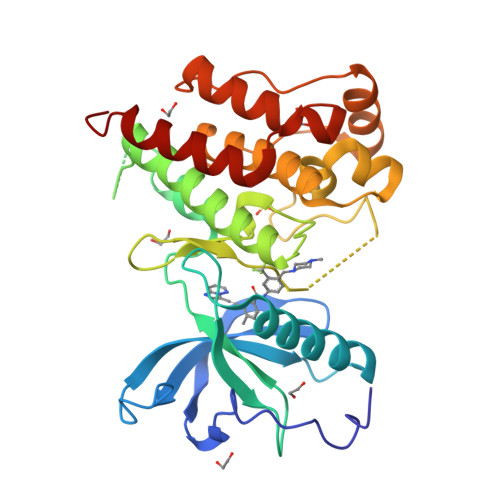
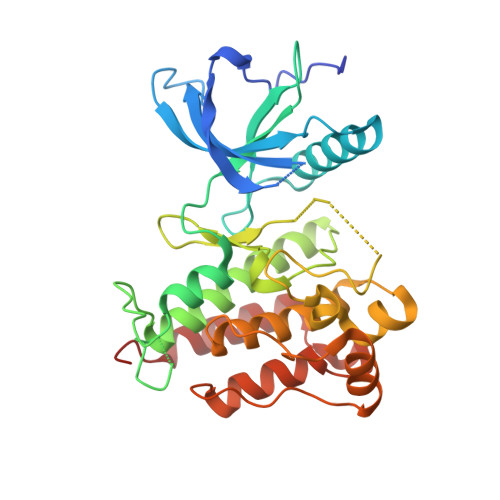
Structural Insights Into Fgfr Kinase Isoform Selectivity: Diverse Binding Modes of Azd4547 and Ponatinib in Complex with Fgfr1 and Fgfr4
Tucker, J.A., Klein, T., Breed, J., Breeze, A.L., Overman, R., Phillips, C., Norman, R.A.(2014) Structure 22: 1764
- PubMed: 25465127
- DOI: https://doi.org/10.1016/j.str.2014.09.019
- Primary Citation of Related Structures:
4UXQ, 4V01, 4V04, 4V05 - PubMed Abstract:
The fibroblast growth factor receptor (FGFR) family of receptor tyrosine kinases has been implicated in a wide variety of cancers. Despite a high level of sequence homology in the ATP-binding site, the majority of reported inhibitors are selective for the FGFR1-3 isoforms and display much reduced potency toward FGFR4, an exception being the Bcr-Abl inhibitor ponatinib. Here we present the crystal structure of the FGFR4 kinase domain and show that both FGFR1 and FGFR4 kinase domains in complex with ponatinib adopt a DFG-out activation loop conformation. Comparison with the structure of FGFR1 in complex with the candidate drug AZD4547, combined with kinetic characterization of the binding of ponatinib and AZD4547 to FGFR1 and FGFR4, sheds light on the observed differences in selectivity profiles and provides a rationale for developing FGFR4-selective inhibitors.
Organizational Affiliation:
Discovery Sciences, AstraZeneca, Mereside, Alderley Park, Macclesfield, Cheshire SK10 4TG, UK.