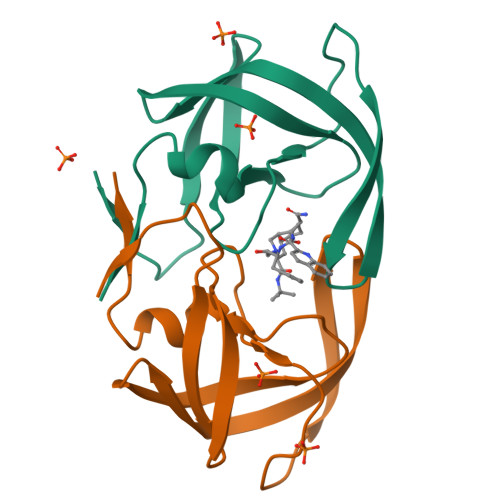
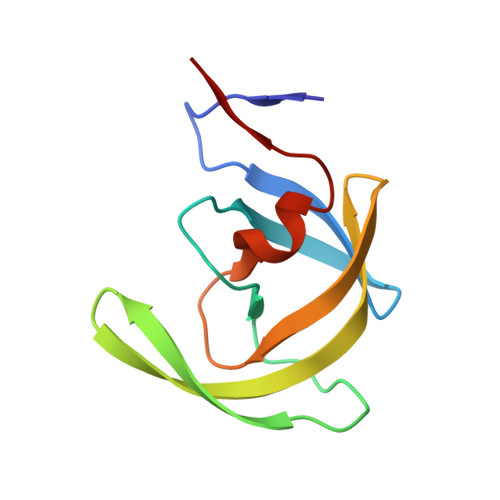
Role of invariant Thr80 in human immunodeficiency virus type 1 protease structure, function, and viral infectivity.
Foulkes, J.E., Prabu-Jeyabalan, M., Cooper, D., Henderson, G.J., Harris, J., Swanstrom, R., Schiffer, C.A.(2006) J Virol 80: 6906-6916
- PubMed: 16809296
- DOI: https://doi.org/10.1128/JVI.01900-05
- Primary Citation of Related Structures:
2FGU, 2FGV - PubMed Abstract:
Sequence variability associated with human immunodeficiency virus type 1 (HIV-1) is useful for inferring structural and/or functional constraints at specific residues within the viral protease. Positions that are invariant even in the presence of drug selection define critically important residues for protease function. While the importance of conserved active-site residues is easily understood, the role of other invariant residues is not. This work focuses on invariant Thr80 at the apex of the P1 loop of HIV-1, HIV-2, and simian immunodeficiency virus protease. In a previous study, we postulated, on the basis of a molecular dynamics simulation of the unliganded protease, that Thr80 may play a role in the mobility of the flaps of protease. In the present study, both experimental and computational methods were used to study the role of Thr80 in HIV protease. Three protease variants (T80V, T80N, and T80S) were examined for changes in structure, dynamics, enzymatic activity, affinity for protease inhibitors, and viral infectivity. While all three variants were structurally similar to the wild type, only T80S was functionally similar. Both T80V and T80N had decreased the affinity for saquinavir. T80V significantly decreased the ability of the enzyme to cleave a peptide substrate but maintained infectivity, while T80N abolished both activity and viral infectivity. Additionally, T80N decreased the conformational flexibility of the flap region, as observed by simulations of molecular dynamics. Taken together, these data indicate that HIV-1 protease functions best when residue 80 is a small polar residue and that mutations to other amino acids significantly impair enzyme function, possibly by affecting the flexibility of the flap domain.
Organizational Affiliation:
Department of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School, 364 Plantation Street, Worcester, 01605, USA.